Can Carbon-Dioxide Be a Liquid?
Carbon dioxide can be a liquid. Depending on the conditions, carbon dioxide (CO2) can be a solid, gas, or liquid. CO2 will form a liquid when the pressure exerted is above 5.11 atmospheric units, and the temperature falls between 69.88° F-87.8° F.
An atmosphere as a unit refers to the average pressure at roughly sea level in many parts of the world. This means that carbon dioxide cannot exist naturally in a liquid state at sea level. In fact, the pressure would need to be more than five times greater than that of sea level.
States of Matter
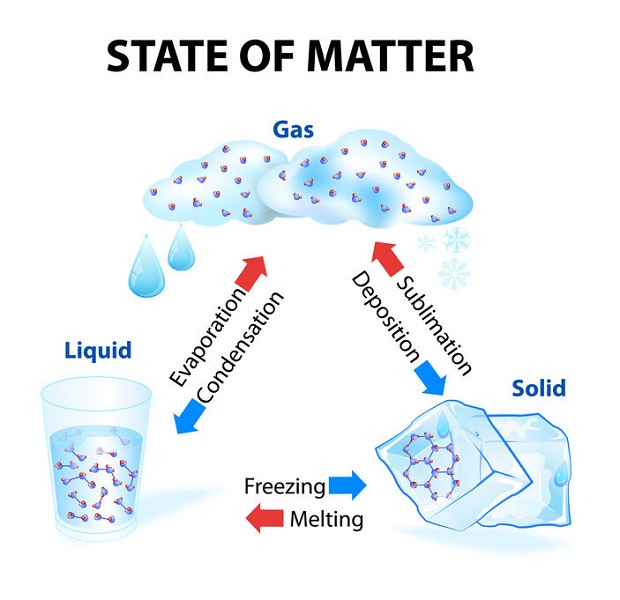
Depending on temperature and pressure, some materials change states from solid to liquid and then to gas. Others, like carbon dioxide, “sublimate”; this means they skip a phase and go straight from solid to gas. This is often the case with carbon dioxide, which in its solid form is called dry ice. Dry ice does not melt into a liquid but rather sublimates into C02 gas.
When Does Carbon Dioxide Become a Liquid?

For gas to liquefy, it must reach critical temperature and critical pressure. The critical temperature is the maximum temperature that gas will liquefy. The critical pressure is the minimum pressure under which gas will liquefy. In the case of carbon dioxide, the critical temperature is 87.8 degrees Fahrenheit. The critical pressure of carbon dioxide is 72.9 atmospheres.
If the temperature is above the critical point, no amount of pressure will cause carbon dioxide to liquefy.
Glossary of Terms

Atmospheric Unit: unit of pressure, equal to the mean atmospheric pressure at sea level. It corresponds to the pressure exerted by a vertical column of mercury (as in a barometer) 760 mm (29.9213 inches) high. One standard atmosphere, which is also referred to as one atmosphere, is equivalent to 101,325 pascals, or newtons of force per square meter (approximately 14.7 pounds per square inch). See also millibar.
Encyclopedia Britannica
Critical Temperature: the temperature at and above which vapor of the substance cannot be liquefied, no matter how much pressure is applied.
Purdue University Department of Chemistry
Critical Pressure: the pressure required to liquefy a gas at its critical temperature.
Purdue University Department of Chemistry
Sublimation: Sublimation is the conversion between the solid and the gaseous phases of matter, with no intermediate liquid stage
USGS
Expert Opinion

“Differences in critical temperatures among gases mean that some gases are easier to liquefy than are others. The critical temperature of carbon dioxide is high enough so that it can be liquefied relatively easily at or near room temperature. By comparison, the critical temperature of nitrogen gas is 126K (-232.6°F [-147°C]) and that of helium is 5.3K (-449.9°F [-267.7°C]). Liquefying gases such as nitrogen and helium obviously present much greater difficulties than does the liquefaction of carbon dioxide.”
Liquefaction of Gases: Critical Temperature and Pressure
JRank Science Encyclopedia