Does Carbon Dioxide Burn?
Carbon dioxide does not burn. Carbon dioxide is a non-flammable gas, but it is the byproduct of burning coal and other fossil fuels. Carbon dioxide is a gas at atmospheric pressures and normal temperatures, occurring naturally in small amounts in the earth’s atmosphere. [1]
Carbon Dioxide Used to Extinguish Fires
Not only is carbon dioxide non-flammable, but it is also used to put out Class B and C fires. Pressurized carbon dioxide in liquid form is used in fire extinguishers for putting out flammable liquids and electrical fires. The carbon dioxide blankets the fire displacing the oxygen fueling the fire. It is also effective at cooling fuel as well. [2]
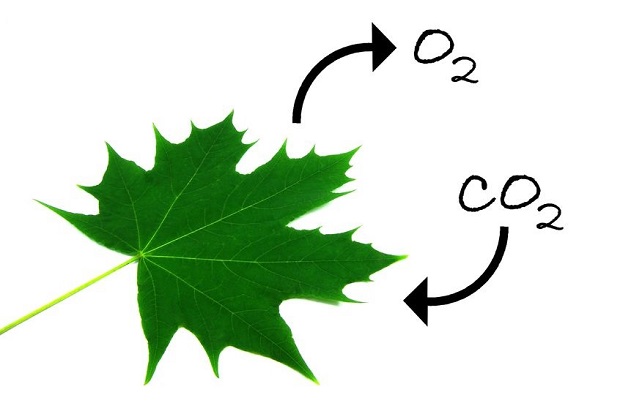
Carbon Dioxide in Respiration
Plants, algae, and certain kinds of bacteria take energy from the sun and manufacture carbohydrates from carbon dioxide and water. This process is called photosynthesis. During photosynthesis, oxygen is released as a waste product. Animals then utilize the oxygen in respiration, exhaling carbon dioxide as a waste product creating a respiration cycle between these life forms. [3]
Other Uses and Sources of Carbon Dioxide
Carbon dioxide is also a byproduct of the fermentation of sugars, such as in alcohol production. It is also released from animal manures and in the exhaust of cars, trucks, and factories.
Frozen carbon dioxide is called dry ice. Dry ice is useful because it freezes at a temperature lower than water, and it does not produce water when it melts as the carbon dioxide returns to a gas state upon melting.
Carbon dioxide is also used to carbonate soft drinks and sparkling waters. It is produced during the fermentation process of beer and champagne, causing bubbles in these beverages. [4]
Resources
[1] Industrial Carbon Dioxide Supply: Liquid & Compressed Gas
[2] The University of South Caroling – Carbon Dioxide Extinguishers
Encyclopedia Britannica – Carbon Dioxide
Glossary of Terms
Carbon Cycle: the biogeochemical cycle by which carbon is exchanged between the biosphere, geosphere, hydrosphere, and atmosphere of the Earth.
Science Daily
Class B Fire: Fire with flammable or combustible liquids as the fuel source.
University of North Carolina Greensboro
Class C Fire: Fire involving electrical equipment.
University of North Carolina Greensboro
Dry Ice: Carbon dioxide in solid form. It is a dense, colorless substance resembling compressed snow that passes directly from solid to vapor at 109.3 °F (78.5 °C) at normal atmospheric pressure. Commonly available in blocks, it is used chiefly to keep foods, vaccines, and other perishable products cold during shipping or storage.
Merriam-Webster Dictionary
Fermentation: Yeast’s anaerobic conversion of sugar to carbon dioxide and alcohol.
Thefreedictionary.com
Expert Opinion
“At room temperatures (20-25 oC), carbon dioxide is an odourless, colourless gas, which is faintly acidic and non-flammable. Carbon dioxide is a molecule with the molecular formula CO2. The linear molecule consists of a carbon atom that is doubly bonded to two oxygen atoms, O=C=O.
Although carbon dioxide mainly consists in the gaseous form, it also has a solid and a liquid form.”
What Is Carbon Dioxide and How Was It Discovered Lenntech