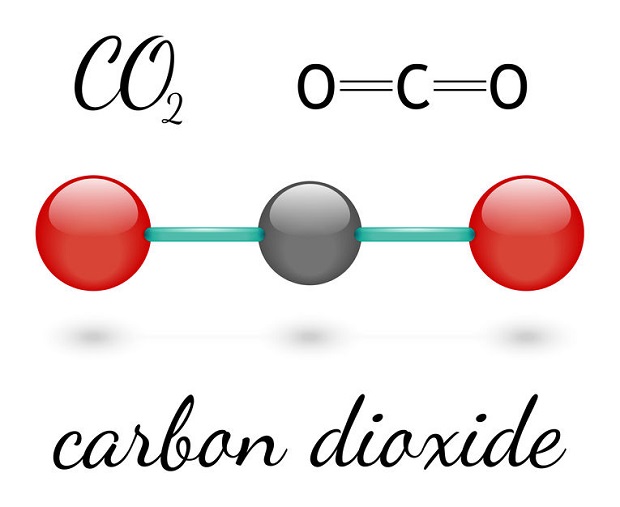
Does Carbon Dioxide Have Mass?
Though it is a gas and may seem weightless, carbon dioxide (CO2) has mass. Like all gasses, liquids, and solids, it is composed of elements found on the periodic table. In particular, a single molecule of carbon dioxide possesses two different elements bonded together. As the name suggests, these elements are a single carbon atom, double bonded to two different oxygen atoms. This is often visually represented as O=C=O.
Periodic Table: A Convenient Tool
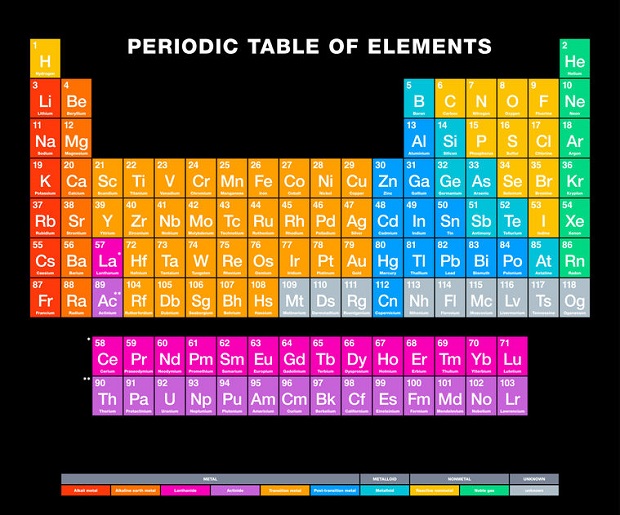
The periodic table is a useful categorization of all the known elements in chemistry. It includes those found in nature and those created by scientists in laboratories. Each element on the periodic table has a specific location and an atomic number. This number was not randomly assigned. It is a measure of the number of protons in the nucleus. A hydrogen atom, for example, has one proton. This means that any element with only one proton in its nucleus is, therefore, hydrogen. A periodic table is a convenient tool for chemists and anyone interested in chemistry. In addition to atomic numbers, the periodic table reveals each element’s chemical properties, electron configurations, and atomic masses.
Elemental Mass Mathematics

The atomic mass is a value that quantifies the sum of the masses of all protons, electrons, and neutrons in a single atom. Though these components are incredibly tiny, they do possess mass. In addition to determining the mass of a single atom, atomic mass can be used to determine the molecular mass or the mass of a molecule. Carbon dioxide has an atomic mass because it is composed of carbon and oxygen. Therefore, it has a molecular mass.
Resources
- LenTech – Carbon Dioxide
- NASA-Cosmic Chemistry: Understanding Elements – The Periodic Table: Atoms, Elements, and Isotopes
- Clinton Community College – Atomic Mass and Mass Number
- Launceston College – Calculating Molecular Mass
- The University of Wisconsin Madison – Department of Chemistry –
- Atomic Structure





