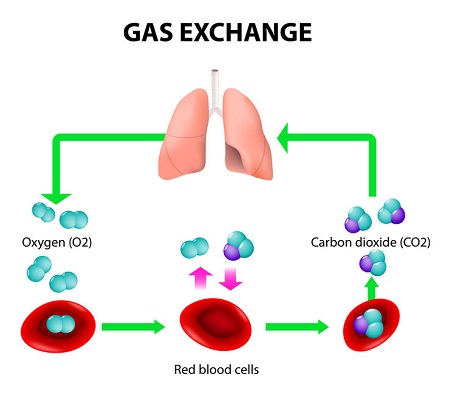
How Does Carbon Dioxide Bind to Hemoglobin?
To bind with hemoglobin, carbon dioxide participates in two biochemical effects, which ultimately create a situation wherein hemoglobin offloads bonded oxygen and then takes on carbon dioxide. Hemoglobin is a protein embedded in red blood cells, which delivers oxygen to tissues and carbon dioxide to the lungs. It creates bonds for transportation purposes but breaks those bonds when it reaches its delivery site.
The Haldane Effect: Regulating Transport
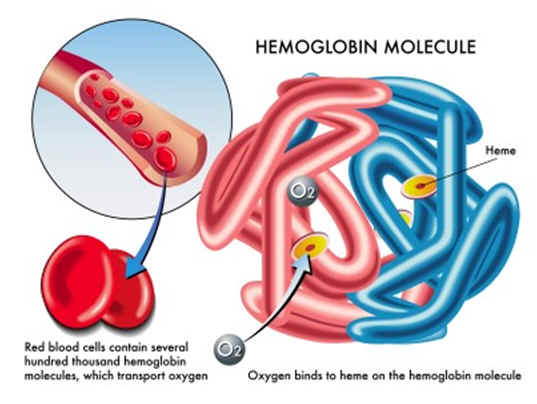
In a general way, hemoglobin can be thought of as a delivery truck with a specific loading capacity. Filling that capacity with oxygen leaves less room for carbon dioxide and vice versa. The body’s biochemistry adds further complexity to this model by increasing or reducing hemoglobin’s carrying affinities due to a particular region’s oxygenation. Regions with high oxygenation result in a reduced affinity for carrying carbon dioxide. Alternatively, regions with low oxygenation increase affinity for carrying carbon dioxide. Known as the Haldane Effect, these altering affinities are vital in delivering oxygen to starved tissues and removing carbon dioxide from the body.
The Bohr Effect: An Inverse Relationship
In addition to the Haldane effect, hemoglobin’s oxygen binding affinity is further influenced by an additional factor. It has an inverse relationship to pH and carbon dioxide concentration, known as the Bohr Effect. Increases in either partial pressures or acidity will lead to oxygen discharging. Afterward, the affinity for removing carbon dioxide increases, and the red blood cells make their return trip.
Bonding
Oxygen bonds directly to the protein’s iron atoms. However, carbon dioxide requires more complicated bonding methods. By dissolving in deoxygenated blood, carbon dioxide participates in a chemical reaction, binding with hemoglobin to form a special compound called carbaminohemoglobin. This allows the transportation of carbon dioxide back to the lungs and subsequent expulsion from the system.
Glossary of Terms
Biochemical Reaction: the process in which two or more molecules (reactants) interact, usually with the help of an enzyme, and produce a product; also, a biochemical reaction may involve the breakdown of a single reactant and the production of multiple products.
San Diego State University
Carbaminohemoglobin: a combination of carbon dioxide and hemoglobin, CO2HHb, being one of the forms in which carbon dioxide exists in the blood.
Dorland’s Medical Dictionary for Health Consumers
Hemoglobin: a protein in red blood cells that carries oxygen. A blood test can tell how much hemoglobin you have in your blood.
Medline Plus
Resources
- Davidson College – Hemoglobin
- Harvard University – Hemoglobin Overview
- The Japanese Journal of Physiology; Tyuma l The Bohr effect and the Haldane effect in human hemoglobin 1984; Volume: 34; No: 2; Pages: 205-216
- East Tennessee State University – The Hemoglobin Page
- Austin Community College – The Bohr Effect





