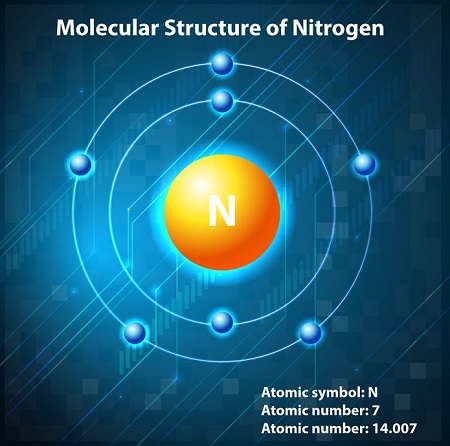
How Many Electrons Are In Nitrogen?
The chemical element nitrogen has a total of 7 electrons. 2 electrons are in shell one while there are 5 in shell two.
Atomic Structure
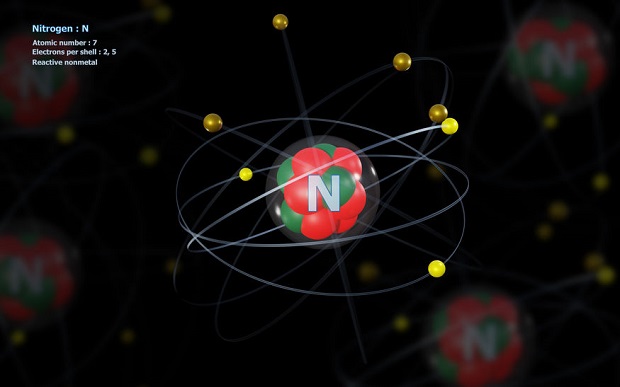
When understanding the electron count in nitrogen, it is often easiest to envision the element in an essential sense. That sense would be an individual atom. According to the Bohr model of the atom, which is still in common usage despite several quantum mechanics advances in thought, the atom has a particular design. The center of an element’s atom is its nucleus, which is made up of protons and neutrons. Around these are several rings or shells, which hold electrons. Each shell has a maximum capacity, which is defined by chemistry. The first shell holds a maximum of two electrons. The second shell holds a maximum of eight electrons. In nitrogen, the first shell is completely filled, and the second shell holds five electrons.
What Are Electrons?
Atomic structure encourages electromagnetic attraction and repulsion. Electrons are negatively charged particles. They maintain their position in the orbitals through attraction to the positive nucleus and repulsion to other electrons. Just as nature abhors a vacuum, electron shells want to be filled to their maximum. Except in cases where electron shells are filled, such as a noble gas like helium, atoms interact with other atoms through their electron shells. For example, they form several types of bonds.
What Is Atomic Bonding
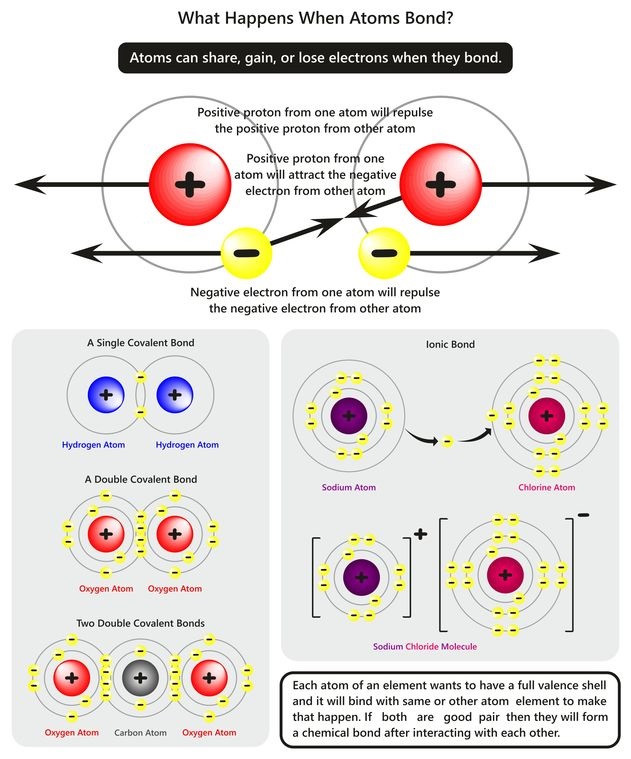
Atoms combine into chemical compounds by developing covalent or ionic bonds. A covalent bond is one where electrons are shared by the two atoms. When such a bond breaks, electrons are neither gained nor lost. In an ionic bond, electrons are lost by one atom and taken by another. Should such a bond break, then one atom will be negatively charged and the other will be positively charged. This is due to the gain or loss of an electron. Nitrogen can form both covalent and ionic bonds.
Resources
Los Alamos National Laboratory – “Periodic Table of Elements: Nitrogen“
NIST – “Periodic Table of the Elements“
Ohio State University – Department of Chemistry and Biochemistry – “Atomic Structure“
University of Illinois – Department of Physics – “What Are Electrons?“
University of California Davis – “Ionic and Covalent Bonds“