How Much Does Water Weigh?
The most widely accepted simple scientific answer to this question is that a gallon of room temperature water weighs 8.33 pounds. What’s more, if water is heated from room temperature, 70°F to its boiling point of 212°F, it will weigh 7.996 pounds, losing .333 pounds.
Why Does Water Weigh Less When Heated?
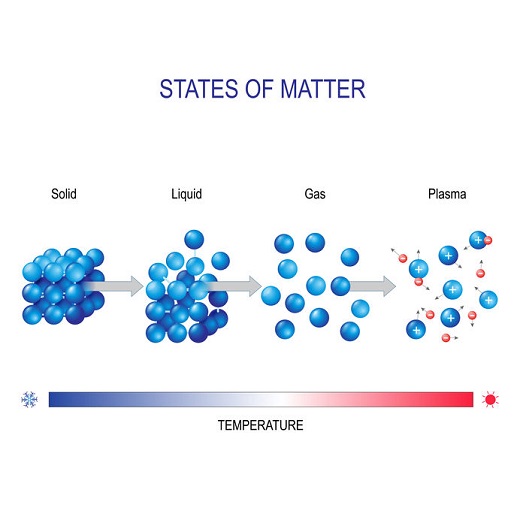
The weight of water is determined by its density. Density is measured by dividing an object’s mass by its volume. When water is heated, the hydrogen and oxygen molecules begin to vibrate more quickly and begin to move farther apart. Though almost negligible, this will increase its volume and decrease its density.
Molecular Weight of Water

The weight of water can also be quantified by its molecular weight, the sum of the atomic weights of the atoms in a single molecule. The scientific elements table abbreviation for water – H2O – refers to the fact that a single molecule is made up of two molecules of hydrogen and one molecule of oxygen. Given that a molecule of hydrogen weighs 1.01 Atomic Mass Unit (AMU) and one molecule of oxygen weighs 16 AMUs, the molecular weight of water is 18.02 AMUs.
Formula: 2(1.01 AMU) +1(16 AMU)=18.02AMU
Note: The atomic weights have been rounded for ease in understanding the concept. The standard atomic weight for hydrogen is 1.00794(2). The standard atomic weight for oxygen is 15.994(3) [4]
Quick Facts Water Chart
- Weight: 8.33 pounds/gallon
- Weight: 62.416 pounds per cubic foot at 32°F
- Weight: 61.998 pounds per cubic foot at 100°F
- Weight: 0.036 pounds/cubic inch
- Density: 1 gram per cubic centimeter (cc) at 39.2°F
- Density: 0.95865 gram per cc at 212°F
Notes of Water Interest

Although waterbeds go in and out of vogue, there is no doubt that they represent one of the most common tactile household experiences of water weight. While a waterbed weighs less per square foot than a refrigerator, the amount of water contained in a king-size waterbed can produce a total weight of approximately 1,500 pounds. Anyone who has ever tried to move a fully filled waterbed without the proper assistance can attest to how cumbersome these items can be.
Finally, the relative density of other physical objects is often filtered through the prism of whether or not they can sink or float in water. There is even a popular reoccurring feature on The Late Show, “Will It Float?”, wherein David Letterman and sidekick Paul Schaeffer try to guess whether or not a particular item will stay on top of the water surface or drop like a stone to the bottom.
Glossary of Terms

Atomic Mass Unit: unit of mass for expressing masses of atoms, molecules, or nuclear particles that is equal to 1/12 of the atomic mass of the most abundant kind of carbon.
Merriam-Webster Dictionary
Cubic Foot: a unit of volume equal to a cube one foot long on each side.
Merriam-Webster Dictionary
Density: the degree of compactness of a substance.
Oxford Dictionary
Molecular Weight: the weight of a molecule, found by adding together the atomic masses of all the atoms it contains; relative molecular mass.
Cambridge Dictionary
Expert Opinion

“Most matter increases in volume when it gets hotter. For example, if an iron rod is heated, it will get longer and fatter, decreasing its density. This happens because the mass of the rod stays the same, but its volume increases. The increase in the volume of matter with increasing temperature is called expansion. When cooled down, most matter decreases in volume and increases in density. This decrease in volume is called contraction.”
Properties of Matter Smithsonian Institution National Science Resource Center
“The density of ice is about 90 percent that of water, but that can vary because ice can contain air, too. That means that about 10 percent of an ice cube (or iceberg) will be above the waterline.”
Water Density US Geological Survey
Resources – Print
Tro, Nivaldo J., and Don Neu. Chemistry in Focus: a Molecular View of Our World. 4th ed. Boston, MA: Brooks/Cole, Cengage Learning, 2009. Page 100 Print.
Resources – Web