Is Carbon Dioxide a Noble Gas?
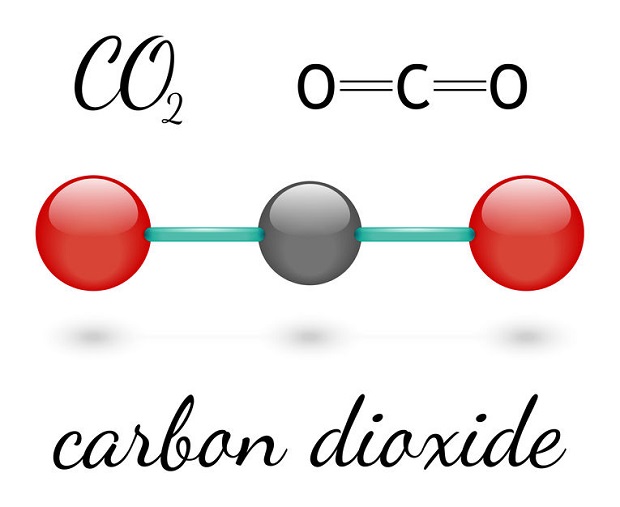
Carbon Dioxide is not a noble gas. Noble gases refer to a set of elements on the far right of the periodic table. Carbon dioxide isn’t a single element. Carbon dioxide is a covalent bond between a single carbon atom and two oxygen atoms. Since it is two separate elements, this inherently prevents carbon dioxide from being a noble gas.
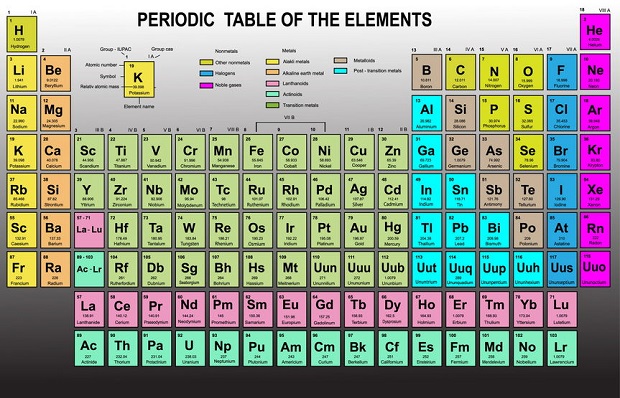
Which Elements Are Noble Gases?

Only six noble gases are on the periodic table of elements located in group 18. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Characteristics of a Noble Gas

Noble gases are what we call chemically inert. An inert substance does not react to other elemental stimuli; it does not undergo chemical reactions. However, this is a loose definition, as scientists have definitively shown that noble gases do, in fact, react with certain catalysts. These are exceptional circumstances. More often than not, scientists and researchers look to use inert gases like those in the noble gas family to prevent reactions such as oxidation (rusting) and hydrolysis.
Characteristics of Carbon Dioxide

You probably know carbon dioxide due to the bad rep it gets. After all, it is one of the most prevalent gases attributed to global warming. You may be surprised to know, though, that carbon dioxide (CO2) is fairly similar to the noble gas family in one main regard: It is also largely chemically inert. For example, CO2 is used during gas metal arc welding (GMAW). The reason is because of CO2’s selective inert nature. It does not react to the weld pool created by arc welding. It is, however, reactive to the arc itself. You may find it interesting that CO2 is often used in the automotive industry for welds because it is so abundant yet closely related to noble gases. This makes CO2 prevalent because it provides an inexpensive alternative to true, noble gases such as argon.
Glossary of Terms

Catalyst: a substance that enables a chemical reaction to proceed at a usually faster rate or under different conditions (as at a lower temperature) than otherwise possible.
Merriam-Webster Dictionary
Covalent bond: used to describe the bonds in compounds that result from the sharing of one or more pairs of electrons.
Purdue University
Hydrolysis: a chemical reaction in which a compound reacts with water to produce other compounds
Reference.com
Oxidation: the process by which iron and steel rust.
Oxford Dictionary
Expert Opinion

“The noble gases Helium, Neon, Argon, Krypton, and Radon are all monatomic. Familiar gases that are triatomic include carbon dioxide, hydrogen sulfide, ozone, and sulfur dioxide. ”
Richard Barrans, Ph.D., M.Ed Why Are Gases Diatomic? US Department of Energy, Ask a Scientist