Is Carbon Dioxide an Element?
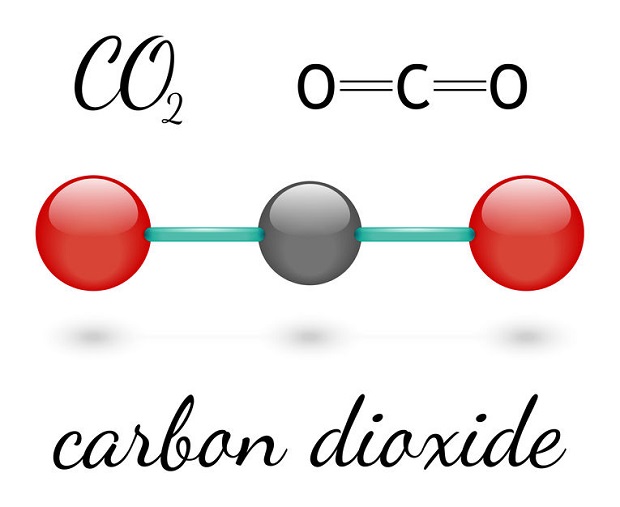
Carbon Dioxide is not an element. Carbon Dioxide (CO2) is a compound. Compounds are made up of individual elements. Carbon Dioxide is made up of two separate elements. There are two atoms of oxygen and a single element of carbon.
Jump Ahead
What Is an Element?
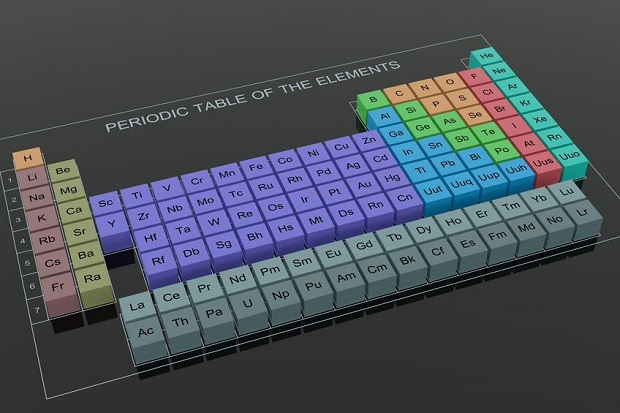
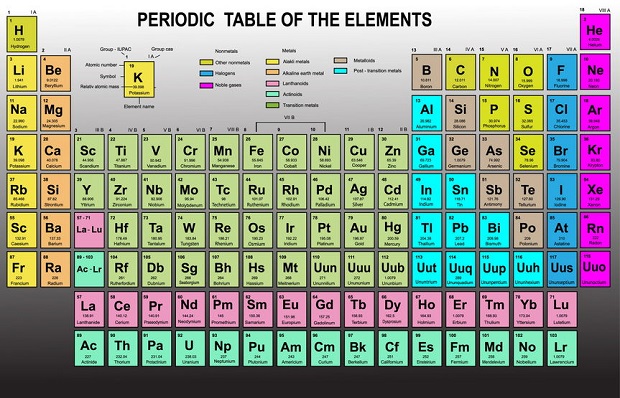
Elements are the fundamental materials of which all matter is composed. An element consists of only one kind of atom, meaning it can not be further broken down into other parts. [1] Currently, 118 known elements are represented by the periodic table of elements that arranges elements by atomic number, electron configuration, and chemical properties. [2] Under standard temperature and pressure, most elements are solid. There are only 11 that are normally in a gaseous state (H2, He, N2, O2, F2, Ne, Cl2, Ar, Kr, Xe, Rn) and only two in a liquid state (Br and Hg). [3]
What Is a Chemical Compound?
A chemical compound is a substance formed when two or more elements are held together by chemical bonds. Elements can combine to form an infinite number of compounds. [4]
The two separate elements that combine to form carbon dioxide are two atoms of oxygen (O) bonded to one carbon atom (C). The chemical formula CO2 represents the compound. [5]
Another familiar compound is H2O or water. The compound H2O consists of one oxygen atom and two hydrogen atoms. [6]
Carbon Dioxide in Nature
Although it isn’t an element, CO2 is extremely abundant in nature. Carbon dioxide is made readily through several different processes. Combustion is one such process. Combustion occurs when organic material is burned in the presence of oxygen, with carbon dioxide, water, and energy being the resulting product. Combustion converts chemical energy into other forms of energy, such as heat. An example of combustion would be the burning of wood. [7]
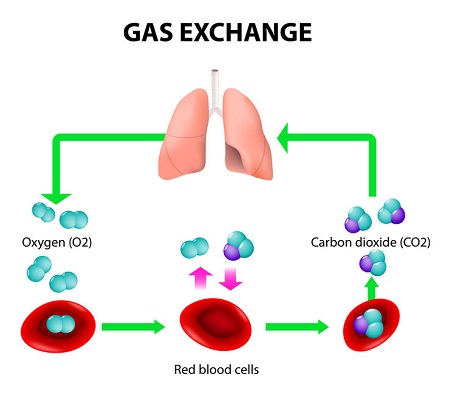
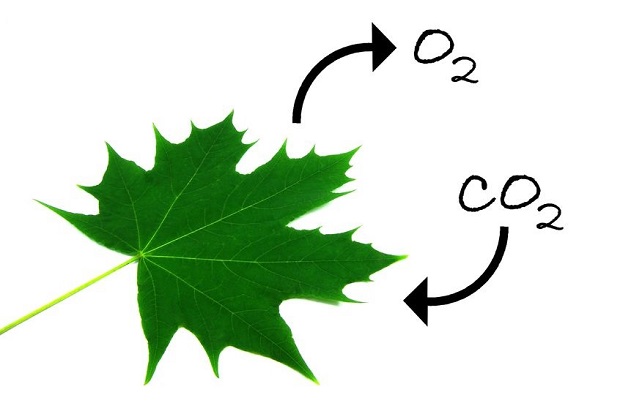
CO2 is also generated through organic chemical reactions. A great example is breathing. When you inhale, you take in oxygen for the numerous chemical reactions happening in your body every second. Every time you exhale, you are expelling carbon dioxide from your lungs. It’s considered a waste product. Plants then take this waste and revert it back to oxygen. [8]
Resources
- [1] Purdue University – General Chemistry – “Elements, Compounds, & Mixtures.”
- [2] American Chemical Society – “Periodic Table of Elements.”
- [3] Louisianna Tech University – “Periodic Table of Elements.“
- [4] Britannica – “Chemical Compound.“
- [5] NIH – National Center for Biotechnology Information – PubChem – “Carbon Dioxide.“
- [6] NIH – National Center for Biotechnology Information – PubChem – “Water.“
- [7] LibreTexts – Chemistry – “Carbon Cycle.“
- [8] Britannica – “The Oxygen Cycle.“